ClariMed, a leading provider of human-centered medical device development and regulatory services, has announced the opening of a state-of-the-art simulated use research facility in Cambridge, UK. This facility, located just 45 minutes from central London and 25 minutes from Stansted Airport, marks a significant expansion in the company’s capabilities, allowing them to conduct advanced research to support the development of user-centered medical devices.
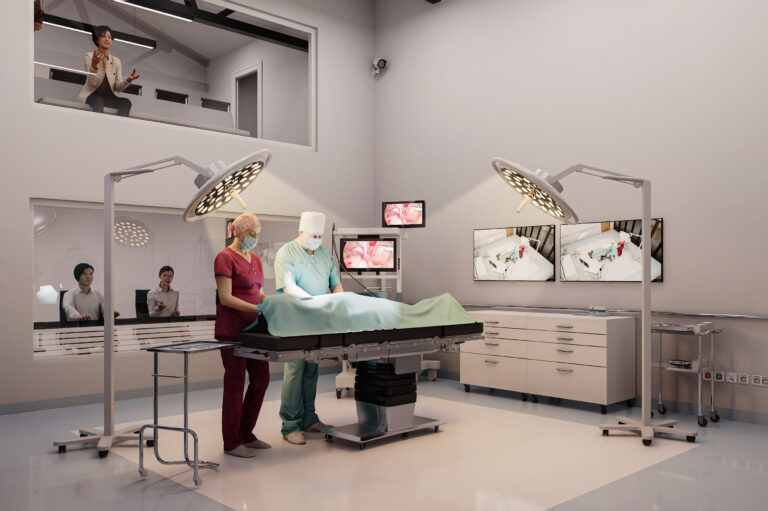
The new purpose-built facility features a host of innovative design elements aimed at enhancing the quality and scope of human factors studies. One of the standout features is the dedicated second-floor observation area, offering exceptional visibility into participant interactions. This design allows clients and researchers to observe the studies with minimal disruption, ensuring more natural user behaviours and more accurate research outcomes.
Julian Dixon, Associate Director of Human Factors and Site Lead at the Cambridge office, commented on the importance of the new space. “This facility represents a significant advancement in our ability to conduct sophisticated usability studies. The elevated observation area provides our clients with unprecedented insight into user interactions, while our enhanced recording capabilities and flexible study spaces allow us to simulate various usage environments with exceptional fidelity.”
Key features of the new facility include:
- Multi-angle recording capabilities for comprehensive interaction analysis
- Flexible study spaces that simulate a variety of healthcare environments
- A dedicated surgical simulation suite
- A dedicated reprocessing room
- A fully functioning ambulance
- An advanced observation suite with real-time data capture capabilities
- Advanced environmental controls for precise testing conditions
The facility’s strategic location also provides access to a diverse participant pool across the UK, facilitating more comprehensive user research. Additionally, its proximity to major transport links makes it easily accessible for international clients and study participants.
Kelley Kendle, CEO of ClariMed, highlighted the significance of the new facility in meeting the evolving needs of global clients. “This investment significantly enhances our ability to serve clients with increasingly complex research needs. The advanced capabilities of the new facility enable us to conduct more sophisticated studies, ultimately leading to the development of safer and more effective medical devices.”
The Cambridge facility is designed to support a wide range of human factors studies, from early-stage concept testing to validation studies. Its flexible configuration allows for testing everything from handheld devices to surgical robots, with specialised areas for simulating home-use and professional healthcare environments.
Jessica Köhne, Senior Human Factors Consultant and Site Lead at ClariMed’s Leeds office, explained the facility’s adaptability: “The ability to recreate authentic usage scenarios across various healthcare settings is invaluable. It helps us understand how medical devices perform in real-world conditions, ensuring our clients can optimise their products for safe and effective use.”
This new facility builds on ClariMed’s recent growth in the UK market, including the opening of an office in Leeds. The expansion further solidifies ClariMed’s commitment to providing comprehensive human factors engineering services to the global medical device industry.